Medical Device Quality Management System
Medical device quality management system. Founded in 2006 Meril is an india based global medical device company. Quality can never be a differentiator if its an afterthought or a box to be checked. Section 51 of Medical Device Act 2012 Act 737 requires a medical device to be registered under the Act before it can be imported exported or placed in the market.
Health Canada Medical Device and Quality Management System Requirements. So why add the burdens of managing IT infrastructure security investments and legacy systems to the mix. A complete end-to-end digital quality management system makes quality at the.
FDA Small Business. The Medical Device Coordination Group MDCG met for their second meeting. Automate as well as manage all quality and compliance processes with one software systemStart by using just the solutions you need now then easily add more as you are readyNo other system is easier to deploy configure and modify.
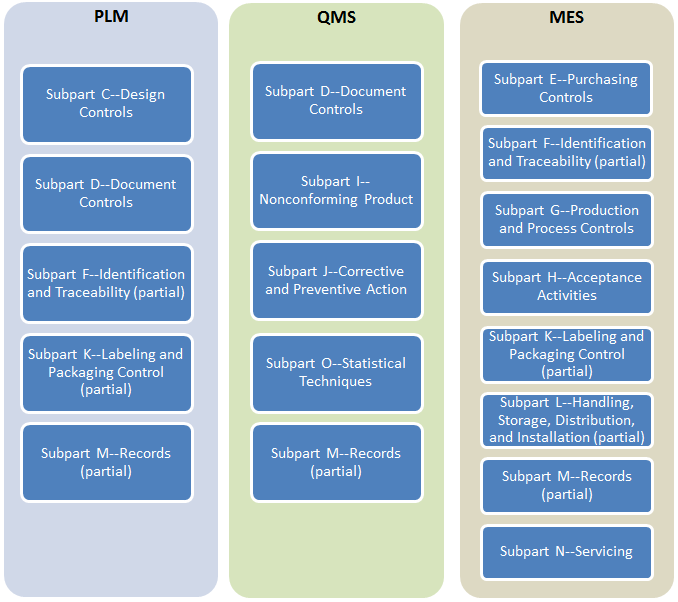
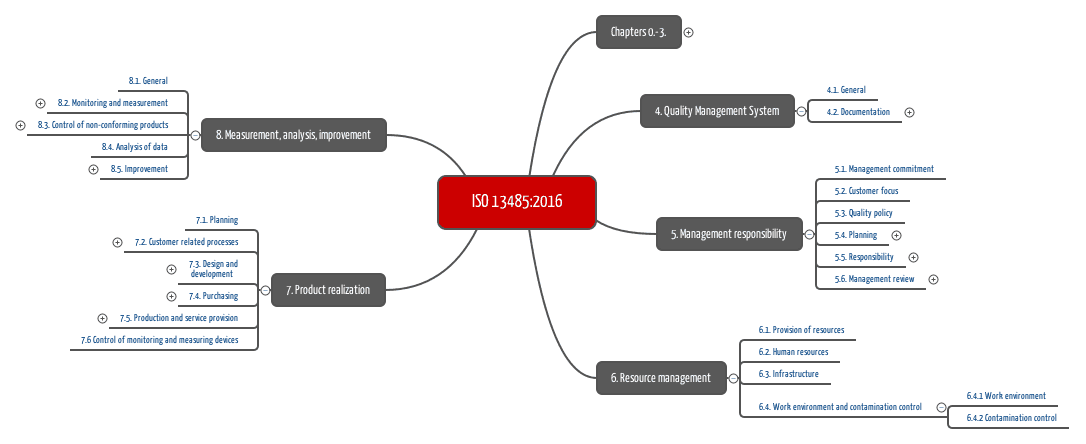
Recognize the impact of public policy on the medical device industry in terms of global market expansion and competitiveness. Expedite time-to-market with a proven medical device manufacturer that prioritizes speed dependability and flexibility. ISO 134852003 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer requirements and regulatory requirements applicable to medical devices and related services.
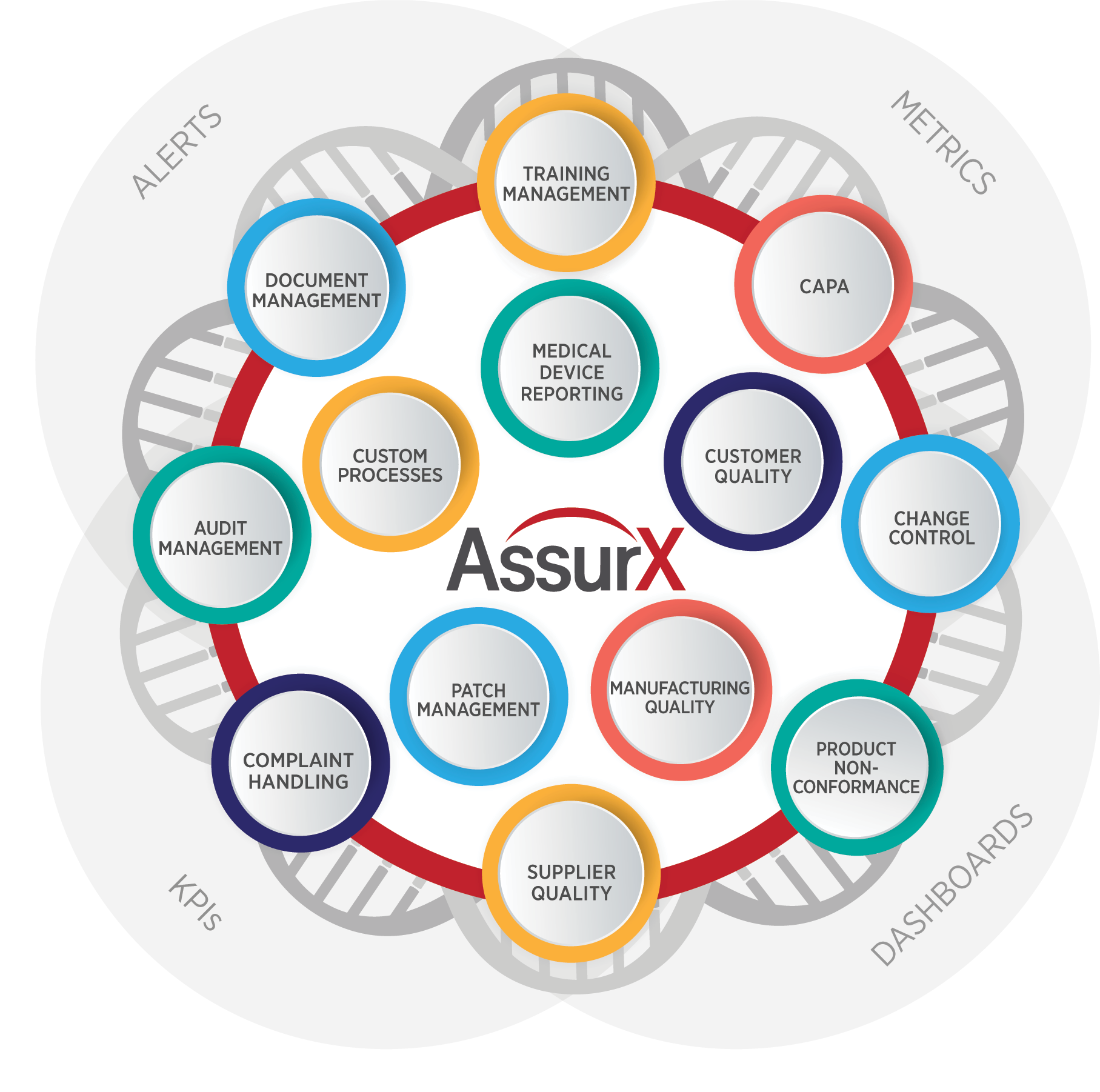
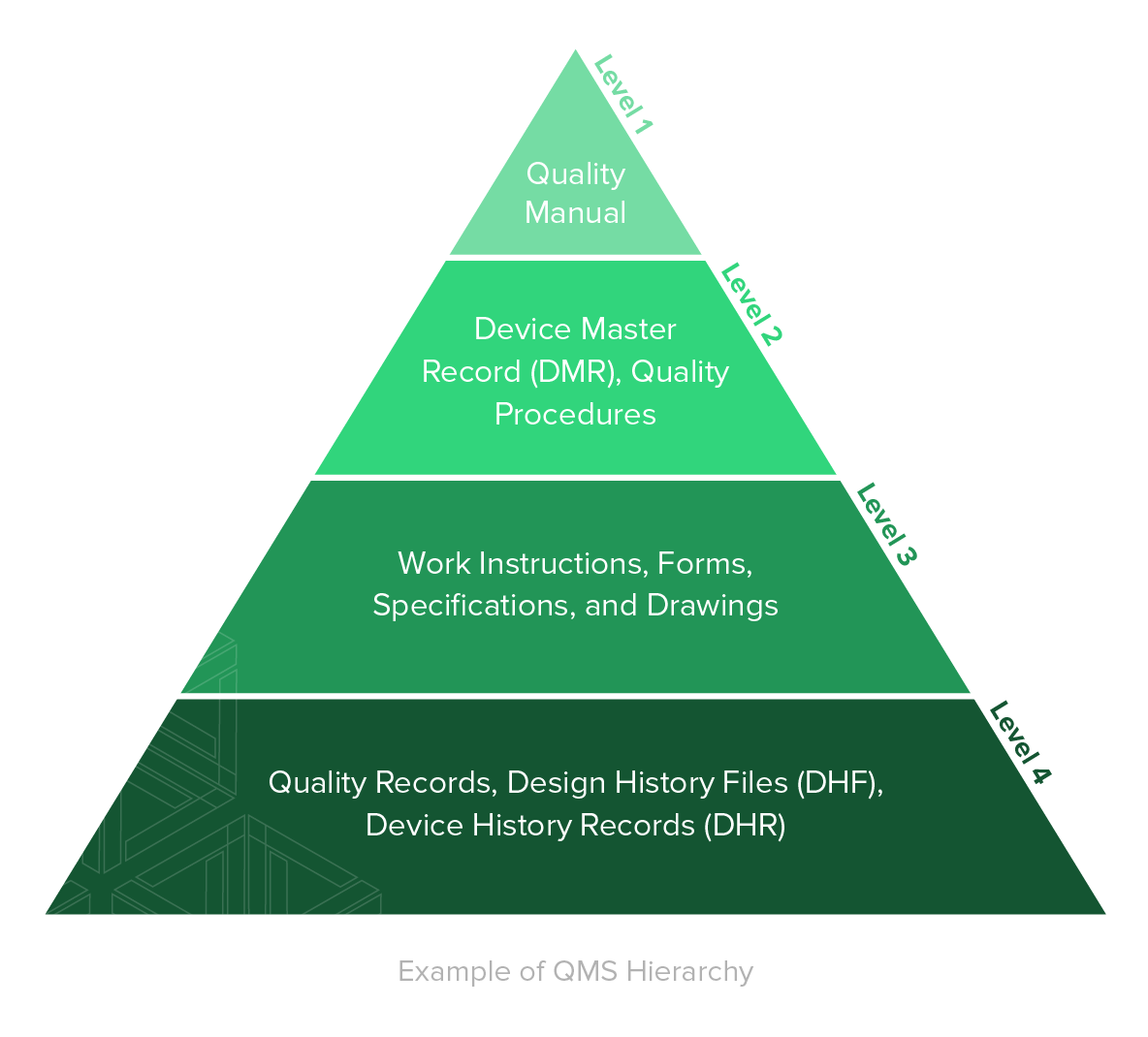
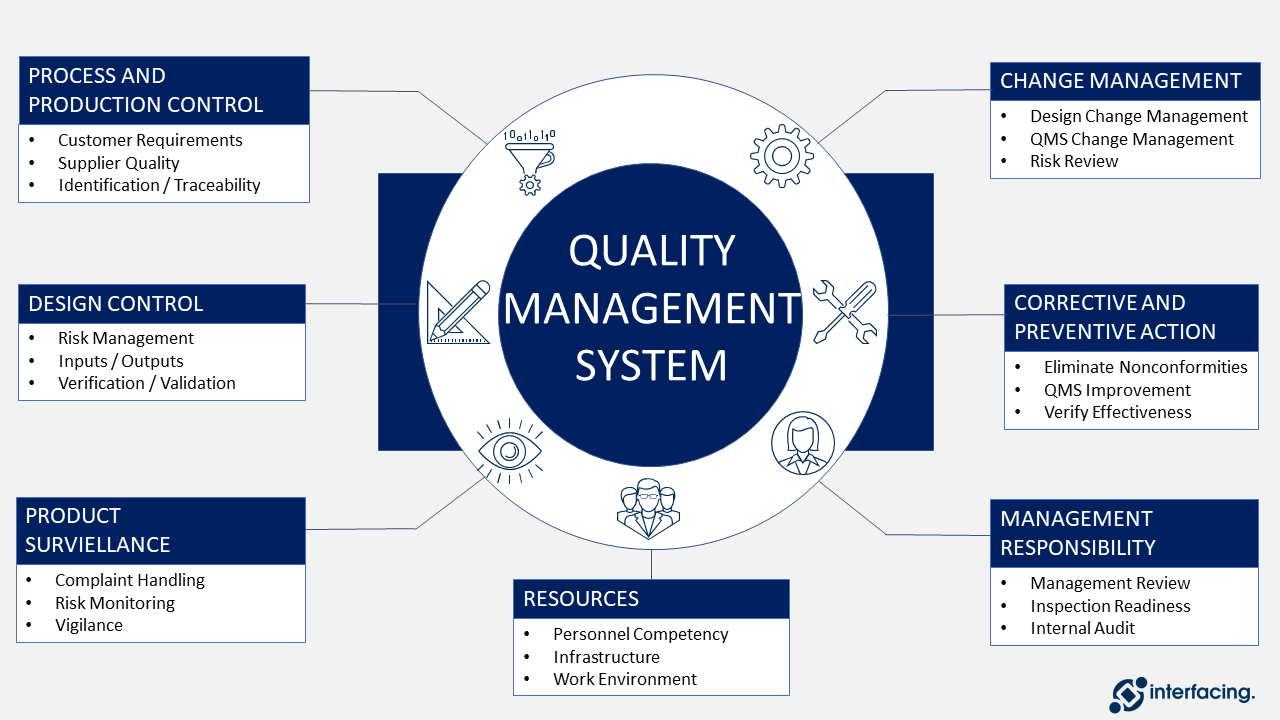
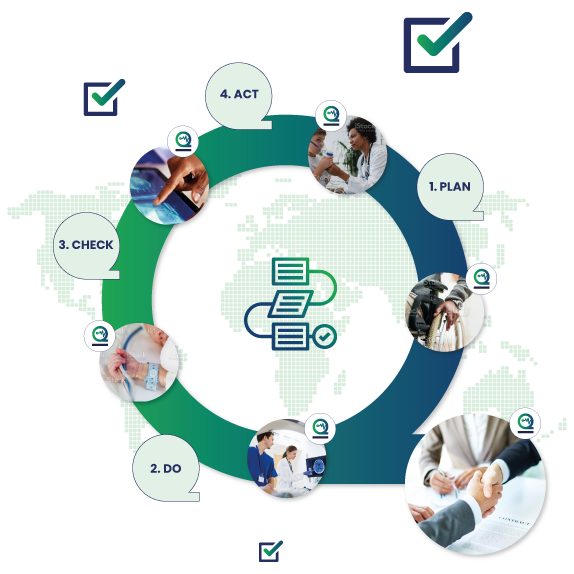
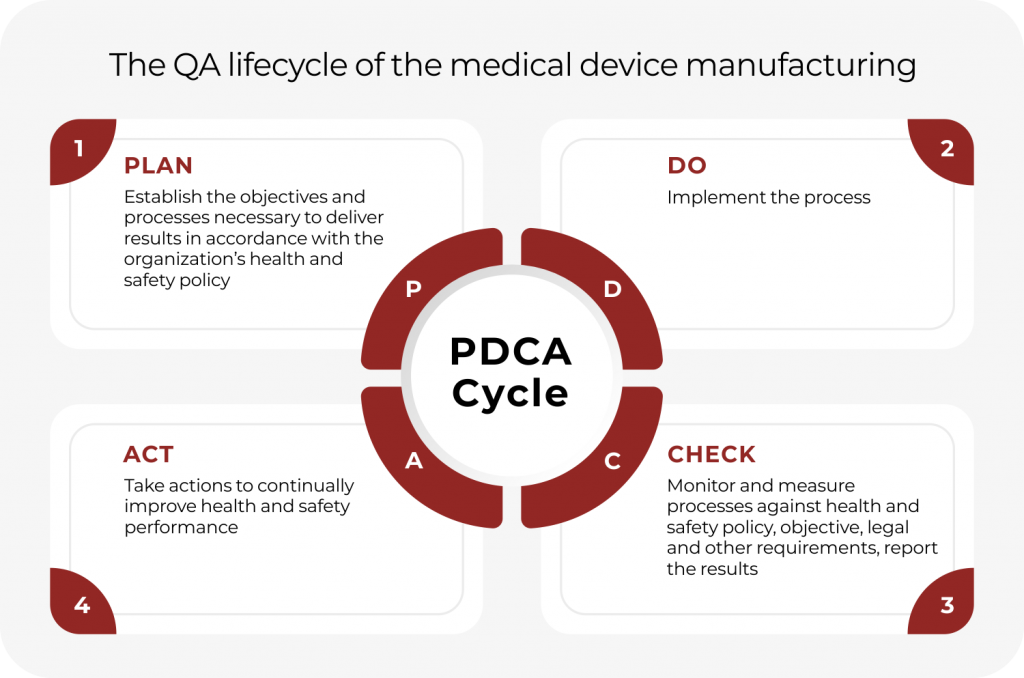
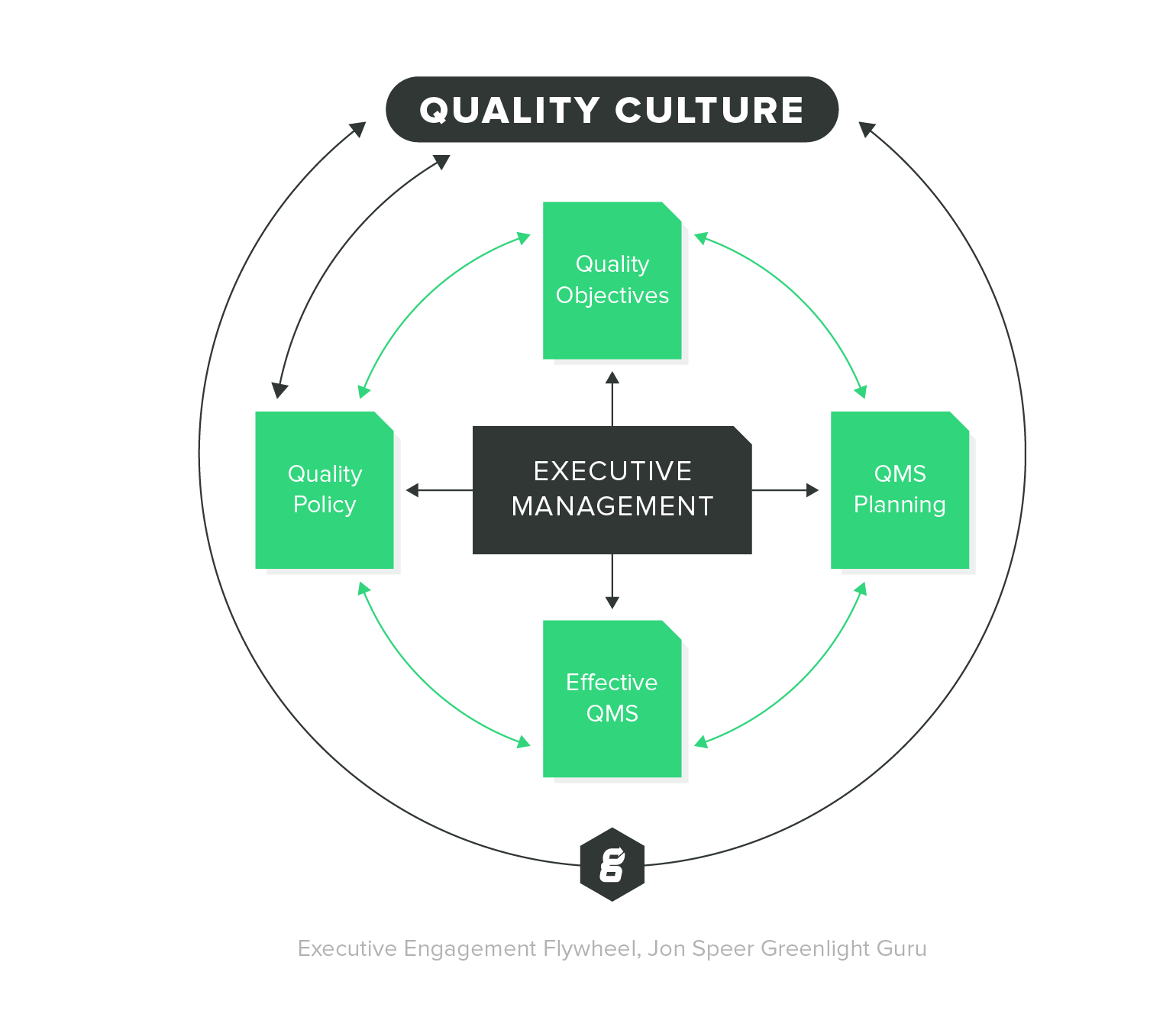
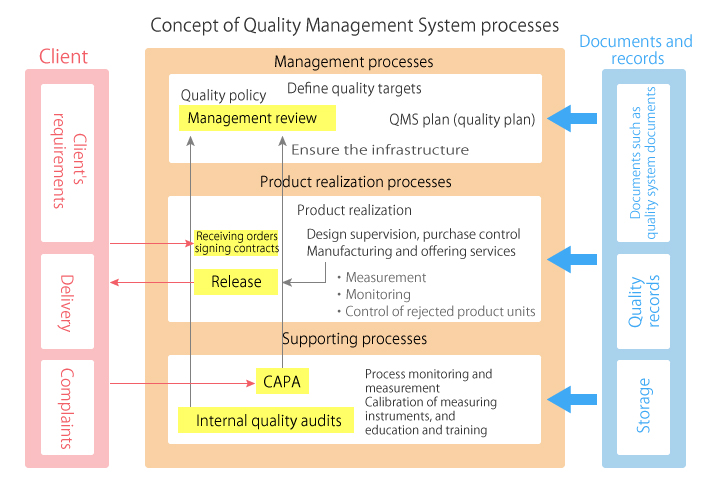
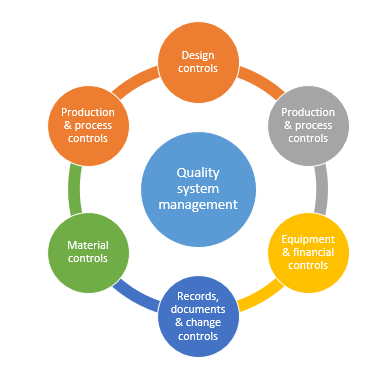
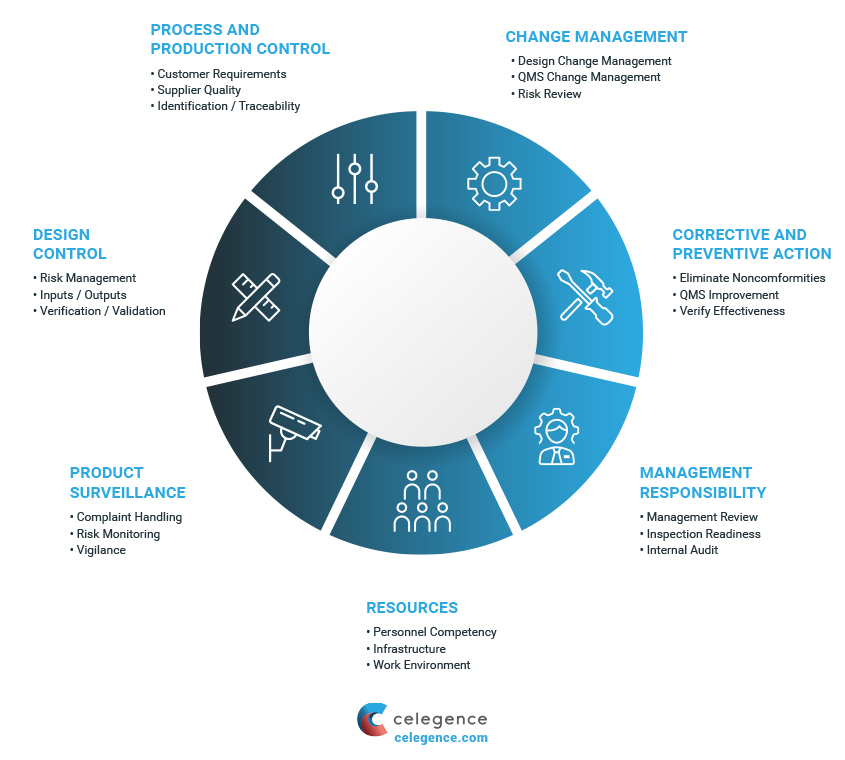
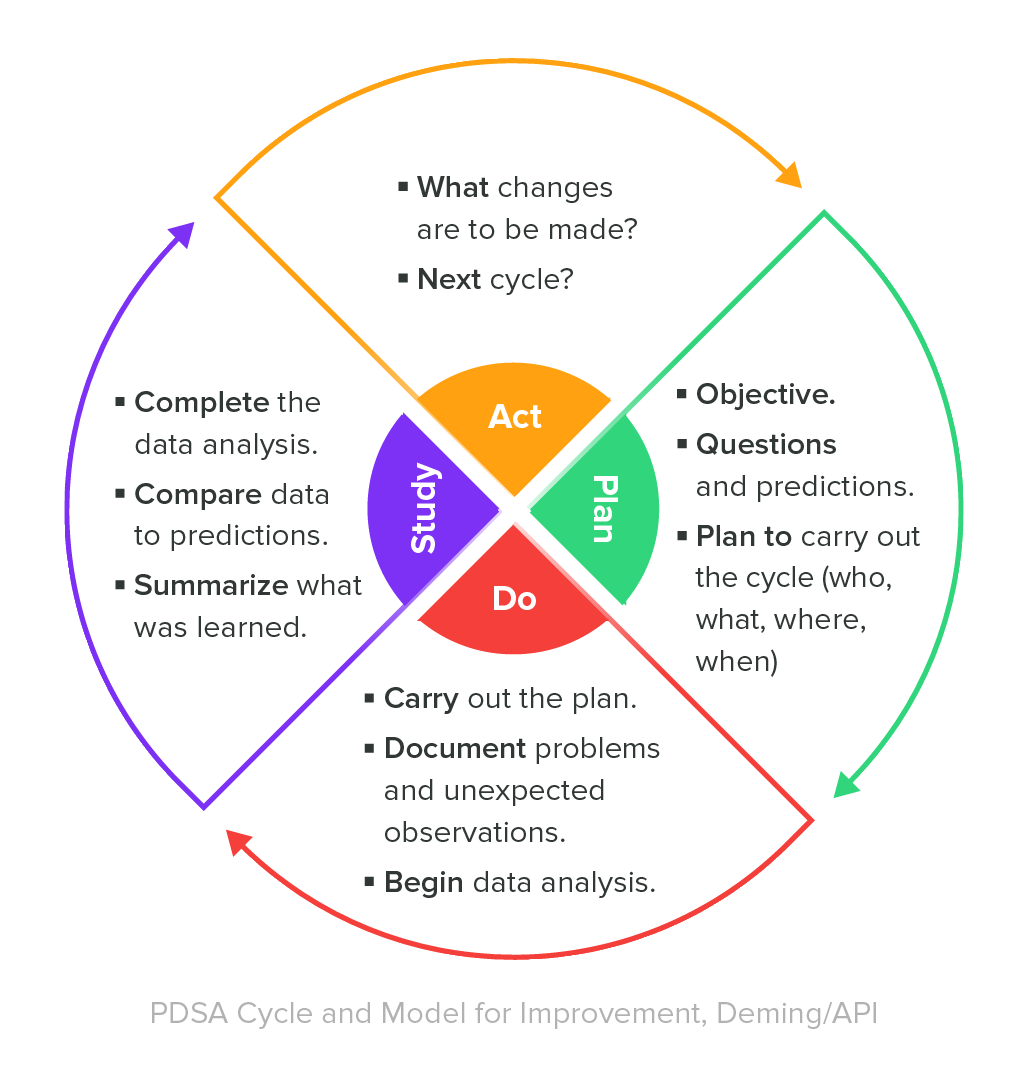
Focuses on product-specific quality aspects of a medical device or device part that may have an impact on the quality safety andor efficacy. Identify the opportunities and challenges in various regulatory markets alongside an analysis on. A quality management system QMS is a collection of business processes focused on consistently meeting customer requirements and enhancing their satisfaction.
The Medical Device Directory reaches key decision makers researching vendors and products including OEMs and component manufacturers including engineers RD Quality Control Materials Managers and Corporate Management Reach this audience by promoting your company in this directory. Regulatory Education for Industry REdI Burlingame CA. It is aligned with an organizations purpose and strategic direction ISO 90012015.
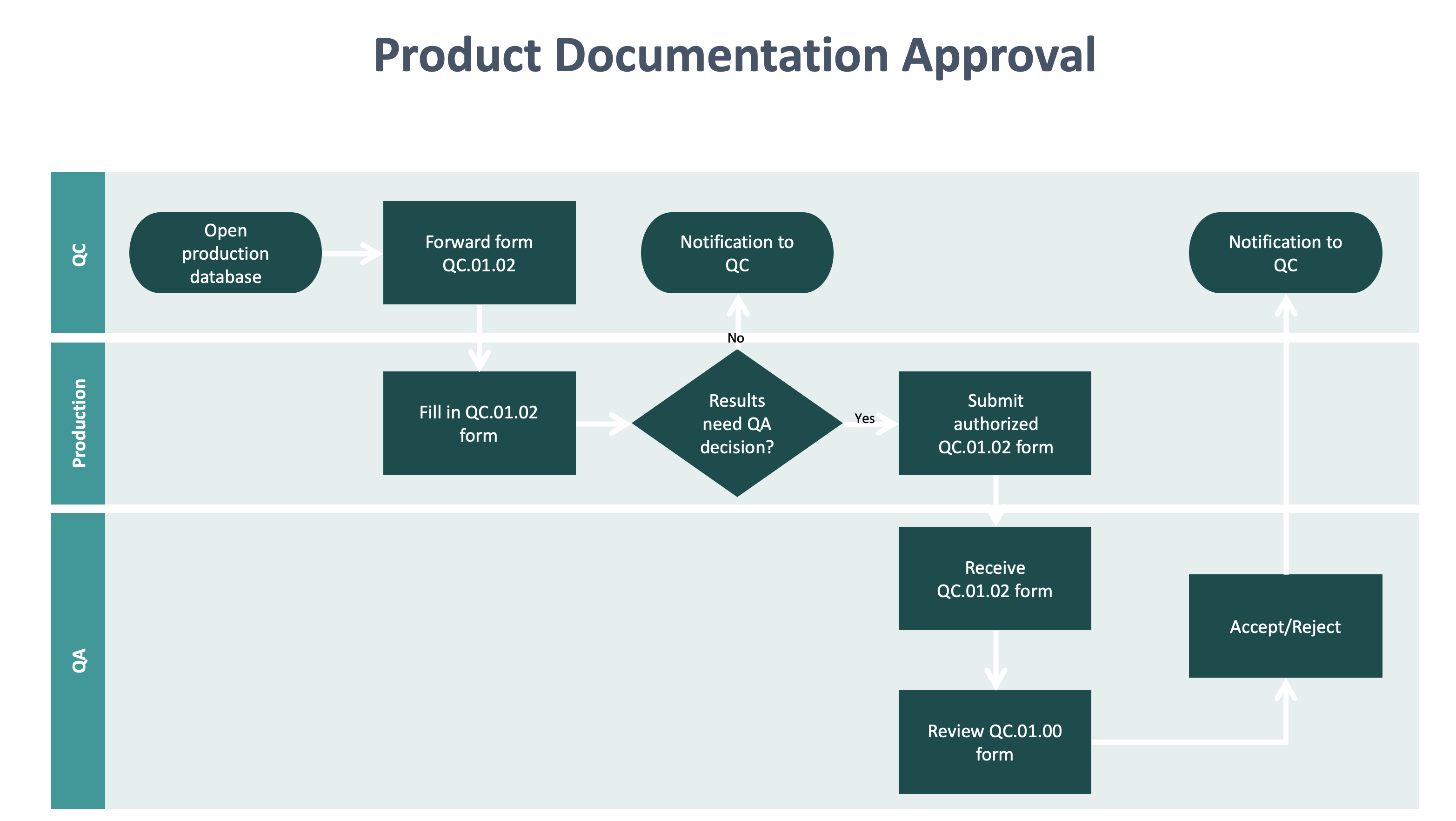
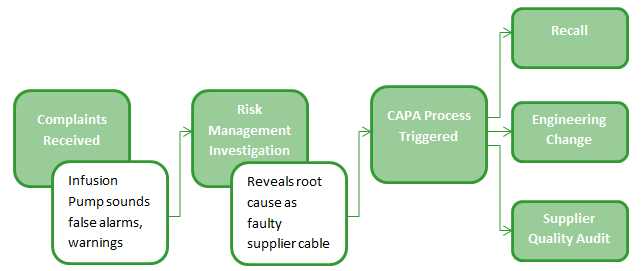
Medical device recalls require speed to ensure patient safety. MasterControls leading cloud-based quality management system QMS is designed.
Section 51 of Medical Device Act 2012 Act 737 requires a medical device to be registered under the Act before it can be imported exported or placed in the market.
Medical device recalls require speed to ensure patient safety. The Medical Device Coordination Group MDCG met for their second meeting. Founded in 2006 Meril is an india based global medical device company. Regulatory requirements are increasingly stringent throughout every step of a products life cycle including service and delivery. Founded in 1998 Sterling Medical Devices specializes in the product design and engineering of medical devices for the healthcare industry. Identify the opportunities and challenges in various regulatory markets alongside an analysis on. Several medical device quality management system regulations have their requirements harmonized around ISO 9001. Join thousands of medical device professionals that take our highly-rated public and customised blended online live virtual class classroom and online courses in risk management design control project management quality management software development and safety for medical devices. Recognize the impact of public policy on the medical device industry in terms of global market expansion and competitiveness.
Identify the opportunities and challenges in various regulatory markets alongside an analysis on. Quality can never be a differentiator if its an afterthought or a box to be checked. When applying for an MDL you will also need to prove that you have a certified ISO 13485 quality management system under the Medical Device Single Audit Program MDSAP which meets the specific requirements of the Canadian Medical Devices Regulations CMDR. Founded in 1998 Sterling Medical Devices specializes in the product design and engineering of medical devices for the healthcare industry. Additional Quality System. Quality System Regulation Overview. Become more confident in medical device product development.



























Post a Comment for "Medical Device Quality Management System"